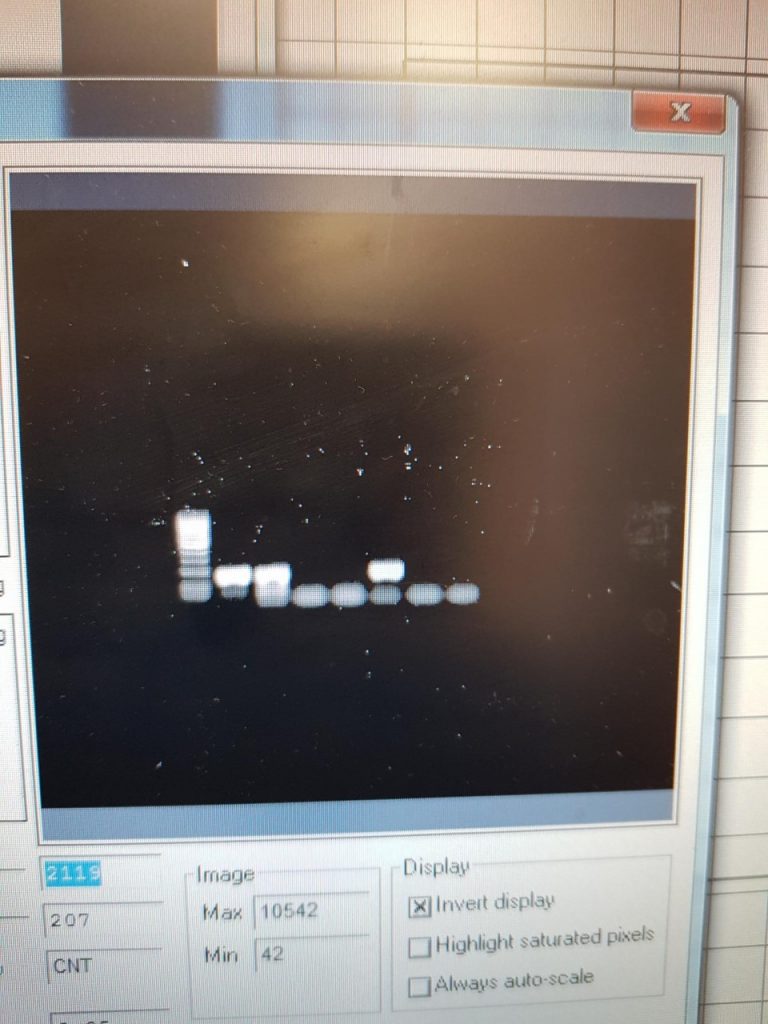
In previous weeks I was carrying out DNA extraction, PCR and gel electrophoresis, these tests were giving me negative results.
Firstly I was not getting sufficient levels of DNA from my faecal samples, to solve this problem I cleaned the faecal samples from the animals by sedimentation and cleaning the samples down till just the eggs and very small amounts of debris were present. Using a microscope and a Pasteur pipette i individually isolated the eggs from this sample and recorded the exact number of eggs present. This technique ensured that I had only Rumen Fluke DNA resent in my sample. The next step was making sure that I was successfully breaking up the eggs so that the DNA was released, this took me over 2 weeks as I was trying different methods such as freeze thaw cycles, boiling, soaking in ethanol, the stool kit, the tissue kit and a hand homogeniser. Eventually we succeeded on breaking up the eggs to relieve the DNA by soaking the sample over night in ethanol and using a sonic beater to use sound waves to break the egg.
The next problem I was having which I have resolved is using the correct PCR settings. My supervisor and I designed primers previously in NUIG, and for these primers to work I would have required different settings for my PCR. Once I ensured I was able to extract the DNA successfully from the rumen fluke eggs i asked my supervisor to order primers from a 2016 research paper on the ITS2 region of rumen fluke, where they also published the settings for us to follow exactly. (However they do not specify the base pair length, so I need to find out the bp length for the 2016 paper primers and the PCR settings that will work with the previously made primers by Galina for me in college )